Abstract
Background
The recent association of cerebral venous thrombosis (CVT) with COVID-19 vaccinations (JAMA; 2021; 325, N Engl J Med 2021; 384) motivated the current review of CVT and MPN. Our objectives were, i) provide an estimation of the incidence of CVT in the context of MPN, followed by a description of clinical phenotype and therapeutic strategies, ii) determine long term outlook in terms of recurrent thromboses, hemorrhage, and survival, iii) identify salient features which distinguish MPN associated from COVID vaccine- related CVT.
Methods
74 consecutive MPN patients with CVT that underwent evaluation at the Mayo Clinic, Rochester MN, USA (n=36), Catholic University, Rome, Italy, (n=23), and University of Florence, Italy (n=15) between 1991 and 2021 were included. The cohort from a previously published multi-center study that included 42 MPN cases with CVT, which were not included in the current study, was used for comparison of observations. Diagnosis of CVT was established with computed tomography or magnetic resonance imaging with venography.
Results
Patient characteristics at time of CVT
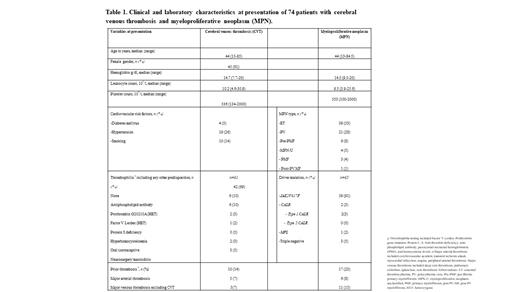
Among 74 patients with CVT and MPN (median age 44 years, range 15-85; 61% females); disease-specific frequencies were 1.3% (39/2,893), 1.2% (21/1,811) and 0.2% (3/1,888) for essential thrombocythemia (ET), polycythemia vera (PV) and primary myelofibrosis (PMF), respectively.
CVT occurred prior to (n=20, 27%, median time to MPN diagnosis 16.5 months), at (n=32, 44%) or after (n=21, 29%, median time to CVT 26 months) MPN diagnosis. 72% of patients presented with headaches, 22% visual changes, 12% nausea/vomiting, 8% neurological deficits, and 6% seizures. Transverse (51%), sagittal (43%) and sigmoid (35%) sinuses were involved with central nervous system hemorrhage in 10 (14%) patients.
MPN phenotype included ET (n=39, 53%), PV (n=21, 28%), pre-fibrotic MF (n=6, 8%), MPN-unclassified (n=4, 5%), PMF (n=3, 4%) and post-PV MF (n=1, 1%). Driver mutation testing was performed in 65 patients: 91% harbored JAK2V617F, 3% CALR type 1, 2% MPL, 5% triple negative; moreover, JAK2V617F was mutated in 27/33 (82%) ET patients. An underlying thrombophilia was identified in 19 (31%) cases. No patient had thrombocytopenia. (Table 1). Notably, one patient received the Ad26.COV2.S vaccine, five days prior to presenting with CVT, not associated with thrombosis in other sites, thrombocytopenia or platelet factor 4 antibodies. A history of thrombosis was documented in 10 (14%) patients with three splanchnic venous events. These observations were similar to those noted in our comparative group from a previously published report that included 42 cases; (ET (n=25, 60%), PV (n=11, 26%), PMF (n=5, 12%); median age 51 years, range 16-84; 55% females; 81% JAK2V617F mutated). Prior thrombosis occurred in 8(19%) patients with four splanchnic venous events.
Treatment for CVT included systemic anticoagulation alone in 27 (36%) patients or in conjunction with aspirin (n=24, 32%), cytoreductive therapy (n=14, 19%), or both aspirin and cytoreduction (n=9, 12%). 5/21 (24%) patients with CVT post MPN diagnosis, were on anticoagulation at the time of CVT.
Outcome following CVT
At a median follow-up of 5.1 years (range; 0.1-28.6), recurrent CVT was documented in 3 (4%) patients; incidence rates for other arterial and venous thromboses and hemorrhage were 11% (2 per 100 patient-years), 9% (1.9 per 100 patient-years) and 14% (3 per 100 patient-years), respectively. 3 of 7 (43%) venous thromboses were splanchnic events. Antithrombotic therapy was ongoing in 53% and 80% of patients with thrombotic recurrences and hemorrhage, respectively. A higher incidence of venous thrombosis was noted in the aforementioned previously published cohort (12 (29%) vs 7 (9%), p=0.01); with 5/12 (42%) splanchnic events. Incidence rates for arterial thrombosis and major hemorrhage were similar. Fibrotic and leukemic transformation occurred in 5 (8%) and 1(1%) patient, respectively, with five (7%) deaths unrelated to CVT.
Conclusions
The current study highlights close association of CVT with JAK2V617F, younger age, and female gender. Clinical features distinguishing COVID vaccine-related from MPN-associated CVT include lower likelihood of concomitant non-CVT venous thromboses with the latter; moreover, the absence of thrombocytopenia resulted in a lower rate of intracerebral hemorrhage in MPN cases; as a result, MPN-CVT was not fatal.
Vannucchi: AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal